These studies found that:

- Inhibition of kappa opioid receptors (KORs) using a short-acting selective antagonist significantly prevented escalation of cocaine intake in rats during chronic exposure and during re-exposure after withdrawal.
- KOR activation using an agonist drug approved for clinical use in Japan significantly reduced oxycodone self-administration in rhesus monkeys.
Manipulation of kappa opioid receptors (KORs) reduced cocaine and opioid self-administration in two NIDA-sponsored studies using animal models, pointing to a likely role of these receptors in the neurobiology underlying drug use disorders. Dr. Marta Valenza, Dr. Kyle A. Windisch, and colleagues at Rockefeller University showed that a selective, short-acting KOR antagonist could lower cocaine self-administration in rats, whereas Dr. Kevin Freeman; his graduate student, C. Austin Zamarripa; and other colleagues at the University of Mississippi Medical Center and from other institutions demonstrated that KOR activation with a KOR agonist could reduce oxycodone self-administration in rhesus monkeys.
Blocking KORs Reduces Cocaine Self-Administration in Rats
There are currently no approved pharmacological treatments for psychostimulant (i.e., cocaine and methamphetamine) use disorders. However, researchers know that in rodents, the levels of KORs and of dynorphins—signaling molecules that bind to KORs—increase in various brain areas after chronic cocaine exposure, making the receptor a potential treatment target. The Rockefeller team previously demonstrated that treating rats with the selective and relatively short-acting KOR antagonist LY2444296 reduced some of the negative withdrawal effects following chronic extended-access cocaine self-administration.
In the new study, the team studied the effects of a related compound, LY2540240, on cocaine intake in rats that could self-administer cocaine for 18 hours per day for 2 weeks, and then again during a single re-exposure session following a 54-hour abstinence period. This design was chosen to mimic experiences of cocaine-dependent individuals following medical or legal interruption of drug taking. Half of the animals were treated with LY2540240 just before their self-administration sessions in the second week and before the re-exposure session.
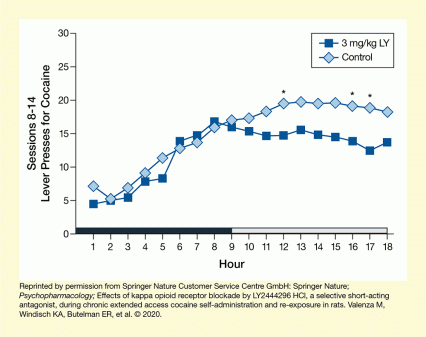
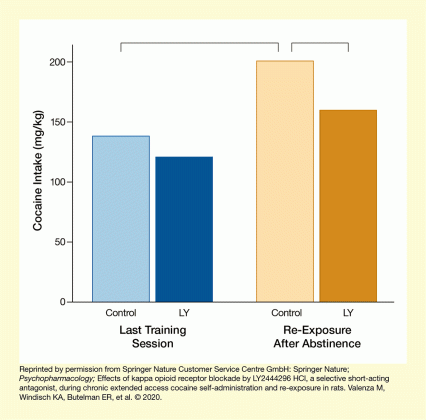
The analyses found that the LY2540240-treated rats administered less cocaine during the second half of each session, but not during the first 9 hours (Figure 1). The researchers concluded that the KOR antagonist may not fully abolish cocaine self-administration but may reduce the escalation of intake that typically occurs during dependence. Additionally, the KOR antagonist significantly reduced cocaine self-administration during the re-exposure session after 54 hours of forced abstinence (Figure 2).
Dr. Valenza says, “This is the first report on the effect of a selective short-acting KOR antagonist in cocaine self-administration and re-exposure after withdrawal.” She emphasizes that the findings are useful for elucidating the neurobiology of addiction, which ultimately is the basis for the development of new treatment approaches. Dr. Windisch agrees, saying, “These studies demonstrate the potential utility of short-acting KOR antagonists for treating some aspects of cocaine dependence, including escalated consumption, negative withdrawal states, and binge re-exposure.” She adds, “The broader implications of these results for the field are that the 18-hour extended access self-administration paradigm is a valuable tool for testing potential psychostimulant treatments aimed at treating drug dependence, including tolerance, escalated consumption, and circadian disruptions.”
KOR Agonists Can Lower Opioid Self-Administration in Monkeys
The significance of KORs in drug use disorders is also supported by the findings of Dr. Freeman and colleagues, who in separate tests combined two KOR agonists—salvinorin A and nalfurafine—with the prescription opioid oxycodone to determine their effects on oxycodone self-administration in rhesus monkeys. Salvinorin A is a typical KOR agonist that produces aversive effects and is associated with side effects that limit its clinical usefulness in preventing drug misuse. Nalfurafine is a KOR agonist already approved for clinical use in Japan to treat intractable itch, and a previous study found that combining nalfurafine with oxycodone can reduce the opioid’s abuse liability and respiratory depression risk in rats while maintaining its pain-relieving effects.
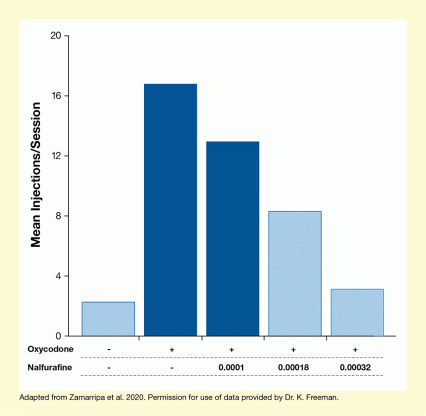
The team now investigated whether the two KOR agonists could reduce oxycodone self-administration in an animal model closer to humans. Rhesus monkeys were trained to self-administer a saline solution (control) or oxycodone with or without salvinorin A or nalfurafine. The results revealed that the animals were highly motivated to self-administer oxycodone. However, when the opioid was mixed with either salvinorin A or nalfurafine, self-administration decreased to control levels (see Figure 3).
Dr. Freeman says, “These results tell us that next-generation KOR agonists like nalfurafine that don’t produce kappa-typical side effects may retain effectiveness at counteracting the misuse-related effects of mu opioid receptor agonists like oxycodone.” He adds, “An oral formulation of an oxycodone/nalfurafine combination appears highly feasible and testable in humans in the near future, given nalfurafine’s current use as an orally administered therapeutic in Japan.”
This work was supported by NIDA grants DA018151, DA039167, DA045011, and DA048586.
- Text Description of Figure 1
-
The figure shows rats’ cocaine self-administration during an 18-hour access period. The horizontal x-axis shows the hours of the self-administration session from 1 to 18. The first 9 hours were during the rats’ dark cycle (indicated by a black bar) and the second 9 hours were during the light cycle (indicated by a white bar). The vertical y-axis represents the average number of lever presses for cocaine per hour during self-administration sessions 8 to 14 on a scale from 0 to 30. Light blue diamonds represent measurements for the control animals that only had access to cocaine. Dark blue squares represent measurements for animals that were treated with 3 mg/kg body weight LY2540240 30 minutes before the self-administration session. During the first 9 hours, both curves run in parallel, with the number of lever presses for both groups increasing from about 5 lever presses after 1 hour to about 17 lever presses after 9 hours. During the second 9 hours, however, the number of lever presses continued to increase slightly for the control animals, reaching about 20 lever presses at 12 hours and remaining approximately at that level for the rest of the session. For the animals treated with LY2540240, in contrast, the number of lever presses declined slightly to about 15 lever presses at 11 hours and remained approximately at that level. Asterisks above the data points at 12, 16, and 17 hours indicate a statistically significant difference between the two groups.
- Text Description of Figure 2
-
The bar chart illustrates the effect of LY2540240 on cocaine self-administration during re-exposure to cocaine after a period of enforced abstinence. The x-axis shows the different sessions. The two blue bars on the left represent the last training session when animals were trained for cocaine self-administration, and the two golden bars on the right represent the re-exposure session after abstinence. Lighter-colored bars represent control animals and darker-colored bars represent animals treated with LY2540240 before the session. The vertical y-axis shows the cocaine intake per 18-hour session on a scale from 0 mg/kg to 200 mg/kg body weight. For the last training session, the light blue bar (left) indicates a cocaine intake of approximately 140 mg/kg for control animals, and the dark blue bar (second from left) indicates a cocaine intake of approximately 120 mg/kg for the LY2540240-treated animals. For the re-exposure session, the light golden bar (second from right) indicates a cocaine intake of approximately 200 mg/kg for the control animals, and the dark golden bar (right) indicates a cocaine intake of approximately 150 mg/kg for the LY2540240-treated animals. Brackets above the bars indicate significant differences between the control animals during the last training session and the re-exposure session and between the control animals and LY2540240-treated animals during the re-exposure session.
- Text Description of Figure 3
-
The bar chart illustrates the effect of increasing amounts of the KOR antagonist nalfurafine on oxycodone self-administration in rhesus monkeys. The horizontal x-axis represents the treatment regimen. Minus signs indicate the absence of oxycodone (top row) and nalfurafine (bottom row). Plus signs in the top row indicate the presence of oxycodone, and numbers in the bottom row indicate the concentration of nalfurafine. The vertical y-axis shows the mean number of injections per session on a scale from 0 to 20. The light blue bar on the left represents control animals that received neither oxycodone nor nalfurafine; these animals had a mean of two injections per session. The second bar from the left represents animals that had access to oxycodone with no added nalfurafine; these animals had a mean of 17 injections per session. As indicated by the dark blue color, this is significantly different from the control animals. The third bar represents animals that had access to oxycodone combined with 0.0001 mg/kg/injection nalfurafine. These animals had a mean of 13 injections per session; again, this was significantly different from the control animals as indicated by the dark blue color. The fourth bar represents animals that had access to oxycodone combined with 0.00018 mg/kg/injection nalfurafine. These animals had a mean of eight injections per session, which was not significantly different from the control animals as indicated by the light blue color. The fifth bar represents animals that had access to oxycodone combined with 0.00032 mg/kg/injection nalfurafine. These animals had a mean of three injections per session, again not significantly different from the control animals, as indicated by the light blue color.
Sources:
- Valenza, M., Windisch, K.A., Butelman, E.R., et al. Effects of Kappa opioid receptor blockade by LY2444296 HCl, a selective short-acting antagonist, during chronic extended access cocaine self-administration and re-exposure in rat. Psychopharmacology (Berl) 237 (4): 1147-1160, 2020.
- Zamarripa, C.A., Naylor, J.E., Huskinson, S.L., et al. Kappa opioid agonists reduce oxycodone self-administration in male rhesus monkeys. Psychopharmacology (Berl) 2020 Jan 31. doi: 10.1007/s00213-020-05473-4. [Epub ahead of print], 2020.