This research found:
- The generation of new nerve cells (i.e., neurogenesis) that occurs during abstinence from methamphetamine in a brain region related to memory and learning may promote relapse-like behavior.
- In rats, inhibition of neurogenesis during abstinence prevented reinstatement of drug use.
See full caption and text description at end of article.
Many people with methamphetamine and other drug use disorders relapse after a period of abstinence, especially if they return to places where they previously used the drug. This phenomenon is known as context-driven relapse. Two studies by Ms. Melissa H. Galinato and colleagues at the University of California, San Diego, and other institutions have shed light on a mechanism that may contribute to context-driven relapse to methamphetamine use.
“New findings from our lab show that neurogenesis—the generation of new neurons in the adult brain—in the hippocampus may strengthen memories tied to drug-seeking behavior in rodents with methamphetamine addiction-like behavior,” says Dr. Chitra Mandyam, senior investigator of the two studies. These findings suggest new approaches for reducing relapse risk. Says Dr. Mandyam, “We also demonstrated that inhibiting neurogenesis during abstinence with a small synthetic molecule prevented context-driven drug-seeking.”
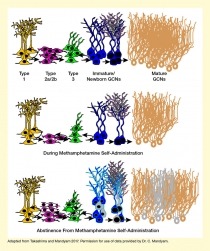
Neurogenesis can occur even during adulthood and is an essential process for learning. For example, newly formed circuits may link rewards, such as food or a drug, to reward-associated memories, such as the location where the reward was received. Earlier studies in rodents had found that methamphetamine administration reduced neurogenesis in the hippocampus, particularly in a region called the dentate gyrus, which has been implicated in memory processing, storage, and retrieval. Abstinence after high methamphetamine self-administration, in contrast, increased neurogenesis in the animals’ hippocampus. However, the newborn cells (progenitors) showed some abnormal characteristics compared with those in animals never exposed to the drug (see Figure 1). Dr. Mandyam and colleagues previously showed that this increased neurogenesis of aberrant progenitor cells was tied to a higher risk of drug reinstatement.
See full caption and text description at end of article.
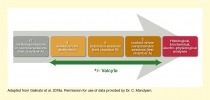
In the new studies, Ms. Galinato, Dr. Mandyam, and colleagues investigated the impact of abstinence-driven neurogenesis in more detail. In the first set of experiments, the research team used genetically modified rats in which adult neurogenesis in the dentate gyrus could be specifically inhibited with the antiviral drug valganciclovir (Valcyte). The experiment included four stages: a 17-day self-administration phase, during which the rats were trained to self-administer methamphetamine or, as a control, sucrose; a 22-day forced abstinence phase; a 6-day extinction phase; and a 2-day reinstatement phase (see Figure 2). About half of the animals were treated with Valcyte from the end of the self-administration phase to inhibit neurogenesis. After the reinstatement tests, the researchers conducted various histological, biochemical, and electrophysiological analyses on the animals.
Rats with intact abstinence-driven neurogenesis exhibited greater methamphetamine seeking during extinction and reinstatement than did the Valcyte-treated animals. This suggests that the formation of new progenitor cells during abstinence assisted with the formation of context-driven memory and was necessary for drug-seeking behavior. Conversely, blocking neurogenesis erased context-driven drug memory in these rats.
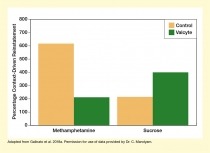
The effect of neurogenesis on drug seeking seemed to be specific to drugs of abuse, because Valcyte treatment did not reduce reinstatement in animals trained for sucrose self-administration (see Figure 3). This specificity raises hopes that targeting neurogenesis may be a viable approach for reducing risk of relapse to drug use.
Additional biochemical and electrophysiological analyses identified some mechanisms through which abstinence-driven neurogenesis may aid memory formation. For example, animals with intact abstinence-induced neurogenesis showed changes in the levels of a protein called CaMKII in the dentate gyrus. CaMKII is associated with the formation of new neural connections (i.e., plasticity), which occurs with memory and learning. These changes were not seen when neurogenesis was blocked with Valcyte treatment. Also, animals capable of abstinence-induced neurogenesis showed evidence of abnormally high neuronal function in the dentate gyrus, which may be associated with memory formation that drives drug seeking. Again, Valcyte treatment prevented these changes, and the effect was specific to methamphetamine-administering animals.
To confirm that prevention of neurogenesis can protect against context-driven reinstatement, the research team repeated the experiments with rats that had not been genetically modified. They treated rats that self-administered high or low levels of methamphetamine with a synthetic small molecule called isoxazole-9 during the abstinence phase. Isoxazole-9 is known to alter neurogenesis in the brains of adult rodents. In rats that self-administered high amounts of methamphetamine—but not in those that self-administered low amounts of the drug—isoxazole-9 treatment blocked compulsive-like drug seeking during reinstatement. These behavioral effects again were accompanied by physiological changes in newborn dentate gyrus neurons, such as reduced numbers of progenitor cells, reduced activation and modified structure of newly formed cells, and changes in CaMKII levels as seen in the earlier experiments.
The studies’ results suggest that neurogenesis during abstinence strengthens drug-associated memories that promote context-driven reinstatement of drug use. Inhibition of neurogenesis during abstinence clears the drug-associated memories, thereby reducing reinstatement risk. However, numerous questions remain. For example, it is unclear whether interrupting neurogenesis could also protect against reinstatement for other drugs, and when this disruption must occur to be effective. An earlier study had found that blocking neurogenesis in the hippocampus before animals began drug use actually increased cocaine self-administration. Nevertheless, Dr. Mandyam says, “These studies open avenues for future clinical investigations of synthetic small molecules in preventing relapse in addicted individuals.”
These studies were supported by NIDA grant DA034140.
- Caption and text description of Figure 1
-
Caption - Neurogenesis in the Adult Rat Dentate Gyrus (DG) (Top) In rats without drug exposure, new neurons are born and develop in distinct stages (indicated by different colors). (Middle) During methamphetamine self-administration, fewer neurons are born (magenta cells), resulting in fewer, but normally developing, cells at all stages of neuronal developmental. (Bottom) During abstinence from methamphetamine, more neurons are born (magenta/white cells), but the numbers of cells at some intermediary stages are reduced (e.g., green, dark blue, and gold cells), and some of the newborn cells are abnormal compared with regular neuronal development (gray cells).
Text Description - The figure shows stages of neurogenesis in adult rat dentate gyrus cells under normal conditions (top), during methamphetamine self-administration (middle), and during abstinence from methamphetamine self-administration (bottom). Different colors represent different stages of cell development. Yellow cells (left) represent type 1 cells, magenta (second from left) represent type 2a/2b cells), green cells (middle) represent type 3 cells, blue cells (second from right) represent immature and newborn granule cell neurons (GCNs), and gold cells (right) represent mature GCNs. Magenta/white and gray cells in the bottom panel represent abnormal cells at each of the indicated stages. In the middle panel illustrating neurogenesis during methamphetamine self-administration, there are fewer cells at each stage of development except for type-1 cells. During abstinence from methamphetamine, there are fewer numbers of normal cells as well as abnormal cells during the various developmental stages except for type-1 cells.
- Caption and text description of Figure 2
-
Caption - The experiment included four stages. In the 17-day self-administration phase, the rats learned to self-administer methamphetamine or sucrose pellets by pressing a lever in a particular test chamber. During the 22-day forced abstinence phase, the animals had no access to methamphetamine or sucrose. In the 6-day extinction phase, the rats were placed in a different chamber where lever presses did not lead to methamphetamine or sucrose reward; this eventually caused the animals to stop pressing the lever. During the 2-day context-driven reinstatement phase, the animals were returned to the initial test chamber where lever presses did result in methamphetamine or sucrose reward to determine if they would resume self-administration. Once this phase was completed, histological, biochemical, and electro-physiological analyses were conducted on the animals. During the abstinence, extinction, and reinstatement phases, half of the animals were treated with Valcyte to inhibit neurogenesis.
Text Description - The figure illustrates the experimental design to investigate the role of neurogenesis in context-driven reinstatement. A gray arrow in the background pointing to the right indicates the order of the experimental stages. A dark gray box (left) represents 17 methamphetamine or sucrose self-administration sessions in test chamber A. A light gold box (second from left) represents 3 weeks of forced abstinence. A medium gold box (middle) represents six extinction sessions in test chamber B. A dark gold box (second from right) represents two context-driven reinstatement sessions in test chamber A. A dark red box (right) represents subsequent histological, biochemical, and electro-physiological analyses. A two-way dark green arrow underneath the gold boxes indicates Valcyte administration in some animals during these experimental stages.
- Caption and text description of Figure 3
-
Caption - The researchers measured how often rats pressed a lever to receive methamphetamine or sucrose when they were returned to a cage in which they had previously received the drug/sucrose. Among rats trained to self-administer methamphetamine, those treated with Valcyte (green) showed significantly fewer lever presses than those without Valcyte treatment (gold). However, no significant difference existed between groups in sucrose-seeking behavior. The findings suggest that Valcyte’s inhibition of neurogenesis specifically affects the formation of contextual memories related to drug use.
Text Description - The bar chart illustrates the effects of Valcyte treatment on context-driven reinstatement in rats trained to self-administer methamphetamine (left pair of bars) or sucrose (right pair of bars). Gold bars represent control animals and green bars represent Valcyte-treated animals with inhibited neurogenesis. The vertical y-axis represents the percentage of context-driven reinstatement on a scale from 0 to 800. For rats self-administering methamphetamine, control animals showed about 600 percent context-driven reinstatement, whereas Valcyte-treated animals showed about 200 percent reinstatement. For rats self-administering sucrose, control animals showed about 200 percent reinstatement and Valcyte-treated animals showed about 400 percent reinstatement.
Sources:
- Galinato, M.H., Takashima, Y., Fannon, M.J., et al. Neurogenesis during abstinence is necessary for context-driven methamphetamine-related memory. Journal of Neuroscience 38(8):2029-2042, 2018.
- Galinato, M.H., Lockner, J., Fannon, M.J., et al. A synthetic small molecule isoxazole-9 protects against methamphetamine relapse. Molecular Psychiatry 23(3):629-638, 2018.