This study reported:
- Exposing adolescent rats to THC disrupted normal maturation of a key set of neurons in a brain area that corresponds to the human prefrontal cortex.
- The disruptions produced structural differences that resemble patterns which have been observed in people with addiction and schizophrenia.
A NIDA-supported study may help explain observed links between adolescent marijuana use and vulnerability to psychiatric disorders. The findings indicate that exposure to the drug’s main psychoactive ingredient, Δ9-tetrahydrocannabinol (THC), disrupts normal maturation of pyramidal neurons in the prefrontal cortex (PFC). These neurons are crucial for the performance of the PFC, which mediates decision making, impulse control, and emotional regulation, among other cognitive functions. The study findings show that THC interferes with their development in ways that may increase adolescent cannabis users’ risks for addiction and schizophrenia.
Dr. Yasmin L. Hurd, senior investigator on the study, says, “Elucidating the links between THC and potential psychiatric morbidity may have great public health significance. Our findings provide important neurobiological targets of focus for further research.”
THC Effects on Neuron Structure
Dr. Michael Miller, Dr. Hurd, and colleagues at the Icahn School of Medicine examined THC’s effects on pyramidal neurons in the rat version of the PFC, called the prelimbic cortex (PLC). In the PLC, as in the PFC, pyramidal neurons undergo a dual process of development during the transition from adolescent to adult neuronal networks. They increase and extend their dendrites, the filaments that collect incoming signals from throughout the brain and conduct them to the neuron body for processing. They also prune away little-used dendritic spines, the structures on dendrites where the incoming signals arrive. Together, these processes strengthen and extend neuronal circuits that have proven useful to the adolescent and increase circuit efficiency by removing excess signal inputs. Once dendrite growth and pruning are complete, the brain is fully mature.
The research team injected male rats three times each week during the period of their adolescence, from age 4 weeks to 7 weeks. The injections produced serum THC levels in the rats similar to those that cause acute cannabis intoxication in humans. The researchers collected brain tissue from some animals 1 day after the last injection (equivalent to late adolescence), and from others 2 weeks after the last injection (equivalent to young adulthood).
Microscopic analyses revealed that PLC neurons of THC-exposed young adult animals had, compared with unexposed animals, an altered dendritic tree with sparser branches (Figure 1). The exposed animals also had fewer dendritic spines in both late adolescence and early adulthood (Figure 2). This latter finding suggests that THC exposure had accelerated pruning, possibly removing some spines that otherwise would have persisted and been incorporated into the animals’ adult neurocircuitry. The changes observed in the THC-exposed PLC neurons resembled those previously reported in animal models of various psychiatric disorders.
THC Effects on Gene Expression
See full text description at end of article.
The researchers also showed that THC exposure in adolescence was associated with changes in gene expression, including of genes with roles in neuron growth and structure. In this research, they used a novel approach called laser capture microdissection and next-generation RNA sequencing to capture and analyze genetic expression in individual pyramidal neurons in a specific area of the PLC (cortical layer II/III). They found that the activity of genes which influence cytoskeletal activity, dendritic branching, and synaptic plasticity differed between the THC-exposed and control animals. The changes observed in the THC-exposed animals were similar to those seen in gene networks that have been linked to psychiatric diseases such as schizophrenia and mood disorders. THC exposure also altered expression of genes involved in epigenetic regulation, suggesting that exposure in adolescence could have continuing effects on neuronal gene expression and function later in life.
Dr. Hurd and colleagues concluded that adolescent THC exposure likely modifies the genetic activity of PFC pyramidal neurons, altering their structure and possibly increasing risk for psychiatric disorders. Their conclusion dovetails with the findings of a recent neuroimaging study of people who regularly used marijuana, which disclosed altered neuronal structure and also pointed to premature pruning in the PFC. Another recent study linked cannabis dependence simultaneously to altered neuronal connections in subcortical brain regions and vulnerability to psychopathology, especially in people who started using the drug early.
Dr. Hurd says, “More research is needed to assess the long-term effects of the THC-induced changes in the PFC as well as THC’s effects on other brain cells. Future studies are needed to confirm a functional impairment in the mesocorticolimbic circuit by adolescent THC exposure and the causal association with specific behaviors relevant to addiction and psychiatric vulnerability. It is also important for future studies to characterize the molecular signature of diverse cortical cells and in different cortical layers to fully identify specific circuits that may be most vulnerable to adolescent THC exposure.”
This study was supported by NIH grants DA038954, DA033660, and DA030359.
- Text Description of Figure 1
-
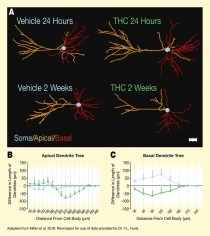
The figure illustrates how THC exposure during adolescence alters dendritic branching of PLC pyramidal neurons during development. Panel A shows graphic illustrations of pyramidal PLC neurons of rats exposed to vehicle (control; left illustrations) or THC (right illustrations) at 24 hours (top) and 2 weeks (bottom) after the last drug administration. Apical dendrites are shown in orange, basal dendrites are shown in red, and the neuron’s cell body (soma) is shown in light blue. At 24 hours after last administration, PLC neurons from vehicle-exposed and THC-exposed animals show similar degrees of dendrite branching. At 2 weeks after last drug administration, dendrite branching in the THC-exposed animals is reduced compared with the vehicle-exposed animals.
The two graphs at the bottom show the difference in length of the dendritic trees between the measurement at 24 hours and the measurement at 2 weeks for the apical dendrite tree (panel B on the left) and the basal dendrite tree (panel C on the right). For both panels, the horizontal x-axis shows the distance from the cell body in μm, on a scale from 0 μm to 480 μm for the apical dendrite tree and from 0 μm to 240 μm for the basal dendrite tree. For both panels, the vertical y-axis shows the difference in length of the dendrites between 24 hours and 2 weeks in μm on a scale from -150 to +150, with a black horizontal line indicating the 0 line; values above this line reflect increased branching complexity and values below this line reflect decreased branching complexity.
In the left panel for the apical dendrite tree, the blue curve representing the vehicle-treated cells remains relatively constant around the 0 line for all distances from the cell body, indicating no substantial change in branching complexity. The green curve representing the THC-treated cells is slightly above the 0 line for distances of 30 μm to 150 μm from the cell body; then decreased for distances of 180 μm to 270 μm, with the greatest difference of about -80 μm; and then increased again to reach the 0 line at a distance of 420 μm from the cell body.
In the right panel for the basal dendrite tree, the blue curve representing the vehicle-treated cells increases for distances of 30 μm to 120 μm from the cell body, reaching a maximum of about +70 μm, and then declines again to reach the 0 line at a distance of 180 μm from the cell body. The green line representing the THC-treated cells decreases for distances of 30 μm to 90 μm from the cell body, reaching a minimum of about -60 μm, before increasing again to reach the 0 line at a distance of 180 μm from the cell body.
- Text Description of Figure 2
-
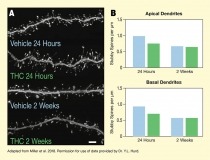
The figure illustrates how adolescent THC exposure causes premature pruning of certain dendritic spines (stubby spines) of PLC pyramidal neurons in rats. Panel A on the left shows microscopic images of basal dendrites of these neurons. The main horizontal gray lines represent the dendrites and the small protrusions above and below them are the dendritic spines. The two top images show dendrites studied 24 hours after the last vehicle (control) and THC administration, respectively, representing animals in late adolescence. Both the dendrite from the vehicle-exposed animal and the dendrite from the THC exposed animal are densely populated with dendritic spines. The two bottom images show dendrites studied 2 weeks after the last vehicle or THC administration, respectively, representing animals in early adulthood. Dendrites from both animals have fewer dendritic spines compared with the dendrites studied after 24 hours.
Panel B on the right shows two bar graphs illustrating the number of stubby spines on dendrites from vehicle-exposed or THC exposed animals at 24 hours or 2 weeks after last drug administration. The top panel shows stubby spines on apical dendrites and the bottom panel shows stubby spines on basal dendrites. Blue bars represent vehicle-exposed animals and green bars represent THC-exposed animals. The vertical y-axis represents the stubby spines per μm of dendrite on a scale from 0 to 1.5. For the apical dendrites, vehicle-exposed animals had approximate 1 stubby spine/μm at 24 hours after last administration and THC-exposed animals had about 0.75 stubby spines/μm. At 2 weeks, both vehicle-exposed and THC-exposed animals had about 0.6 stubby spines/μm. For the basal dendrites, vehicle-exposed animals had about 0.9 stubby spines/μm at 24 hours and THC-exposed animals had about 0.7 stubby spines/μm. At 2 weeks, both vehicle-exposed and THC-exposed animals had about 0.5 stubby spines/μm.
Source:
- Miller, M.L., Chadwick, B., Dickstein, D.L., et al. Adolescent exposure to Δ9-tetrahydrocannabinol alters the transcriptional trajectory and dendritic architecture of prefrontal pyramidal neurons. Molecular Psychiatry 24(4):588-600, 2018.