The National Institute on Drug Abuse (NIDA) is the lead federal agency supporting scientific research on drug use and its consequences. Its mission is to advance science on drug use and addiction and apply that knowledge to improve individual and public health. After decades of research, addiction is now understood to be a chronic, treatable brain disorder from which one can recover. NIDA-supported research has led to the development of effective prevention and treatment interventions, providing hope for the millions of people in the United States diagnosed with substance use disorders (SUDs) and their loved ones.
The Addiction Public Health Crisis
- 20.4 million people in the United States were diagnosed with SUD in the past year1
- Only 10.3 percent of people with past-year SUD received SUD treatment1
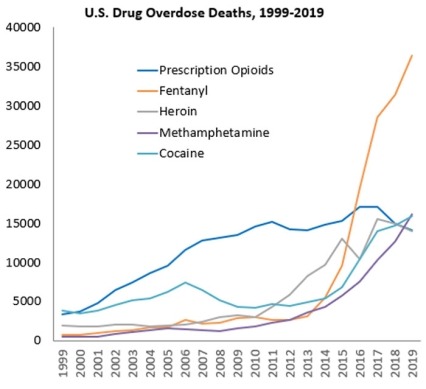
- Nearly 71,000 people died of drug overdoses in 20192
NIDA’s Research Investment
- 363 Full-time equivalents (FTEs)
- 354 FY 2020 New Research Project Grants
- 477 Unique investigators in FY 2020
- 73 Early stage investigators
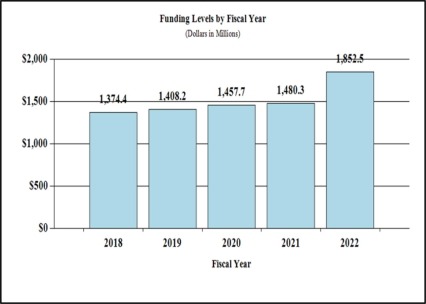
- $266M for FY 2020 HEAL projects
- $10.9M for FY 2020 COVID-19 studies
- $261M for FY 2020 HIV/AIDS projects
- Basic Neuroscience: Understanding how drugs affects the cells and circuits of the brain, how addiction occurs, and how genes and environment affect the brain
- Epidemiology: Monitoring emerging trends in drug use
- Risk and Protective Factors: Identifying the factors that influence drug use, addiction, access to care, and related health outcomes
- Prevention: Developing and testing approaches to mitigate risk factors, promote resilience, and prevent drug use, addiction, and their consequences
- Treatment: Developing and testing medications, devices, and behavioral treatments for addiction and its consequences
- Implementation: Optimizing approaches for scaling-up and enhancing access to evidence-based treatment and prevention strategies
Recent Accomplishments
- Lofexidine: first medication for opioid withdrawal symptoms approved by FDA in 2018.
- ReSET-O: first smartphone app to deliver behavioral treatment for opioid use disorder (OUD) approved by FDA in 2018.
- ADAPT-2 Trial: multi-site clinical trial showed effectiveness of a combination of naltrexone and bupropion in reducing methamphetamine use.
- Tobacco Research and Policy: findings from NIDA-supported studies demonstrating the appeal of flavored e-cigarettes to youth informed FDA’s 2020 policy prioritizing enforcement against certain unauthorized vaping products.
- NIDA Clinical Trials Network: with HEAL funds, added 5 new research nodes and supported the development of 26 new protocols to develop and test substance use interventions.
Current Activities
Image

- Adolescent Brain Cognitive Development Study: the largest long-term study of brain development and child health ever conducted in the United States is continuing to track brain and behavioral development of nearly 12,000 9-10 year-olds through early adulthood.
- NIH HEAL InitiativeSM: administering over $250M/year to prevent and treat opioid misuse, addiction, and overdose, including studies to develop and test new interventions and effective strategies for implementing proven interventions across settings.
- Medications Development: supporting over 40 grants in FY 2020 to develop medications and other treatments for OUD and over 25 to develop treatments for stimulant use disorders.
- Cannabis Research: supporting over 250 studies in FY 2019 to understand the basic science, health effects, and therapeutic potential of cannabis and cannabinoids.
- NIDA Technology Portfolio: advancing research on cutting-edge technologies for preventing and treating substance misuse, addiction, and overdose.
- COVID-19 and SUD: supporting over 90 studies to understand how COVID-19 may affect people with SUD and child health outcomes.
Future Initiatives
- HEALthy Brain and Child Development (HBCD) Study: participant recruitment for the most comprehensive study of early brain development ever conducted will begin in FY 2022. The study will enroll 7,500 pregnant and postpartum women and follow them and their children through early childhood to determine how maternal drug exposure and other factors influence development.
- HEALing Communities Study: researchers are on track to the deploy the intervention of this multisite implementation study across 67 communities in four states, aimed at reducing opioid overdose deaths by 40 percent in participating communities.
- HEAL Prevention: nine studies focused on preventing opioid use during the transition from high school to early adulthood are completing their pilot phase and transitioning to larger clinical trials.
- Justice Community Opioid Innovation Network: 10 grants and $22 million in FY 2020 to test SUD prevention and treatment interventions will begin data collection.
References
- 2019 National Survey on Drug Use and Health
- 2019 The Centers for Disease Control and Prevention WONDER database