This study found that:
- A specific collection of neurons in the basolateral amygdala, a brain region crucial for affect, was active when mice experienced pain.
- Deactivating this collection of neurons resulted in a loss of negative affective responses to painful stimuli, although the animals still perceived the pain.
Pain, and particularly chronic pain, can make a person’s life miserable. However, the results of a recent NIDA-funded study suggest that pain may not have to be unpleasant. This would be welcome news to those suffering from the emotional burden associated with chronic pain.
To help pain patients, scientists to date have focused mostly on learning how sensory neurons are activated and how these pain signals are transmitted to the brain, in hopes of finding ways to block pain at its source. This strategy has not met with great success, however, and despite decades of study, analgesic drugs still cannot fully eliminate the suffering of chronic pain patients. Additionally, some of these drugs, like opioids, have safety issues and may be misused. Therefore, postdoc Dr. Gregory Corder (now an Assistant Professor at the University of Pennsylvania), graduate student Biafra Ahanonu, and their colleagues at Stanford University decided to pursue a different approach. Dr. Grégory Scherrer, one of the study’s senior investigators (who has since moved to the University of North Carolina at Chapel Hill), explains, “We decided not to look at how the pain neural signals are produced and come into the brain through the nerves and then the spinal cord, but rather tried to understand what the brain was doing with this information to make pain unpleasant.”
The researchers focused on a brain area called the basolateral amygdala (BLA) because damage to this region can cause a rare phenomenon in which a person detects a painful stimulus but does not perceive it as unpleasant. The team exposed mice to different painful stimuli, such as pinpricks or heat, and then used various behavioral, cellular, and molecular methodologies to detect which neurons in the BLA were active when the mice were in pain. These tests identified a specific set of neurons that were activated in response to various painful stimuli, but not to other unpleasant or pleasant stimuli (see Figure 1).
BLA Neurons Mediate the Behavioral Response to Pain
Next, the investigators examined whether preventing these cells from working would affect the animals’ response to pain. Normally, when animals are exposed to a painful stimulus, such as pricking a paw, their response has two components:
- First, the animal will withdraw its paw—a reflex-like physiological response to the pain signals to prevent further harm.
- Second, the animal will begin to lick the paw and run away—a behavioral response indicating that it experiences the pain as unpleasant and is motivated to make pain unpleasantness stop.
When the researchers inhibited the newly identified BLA network of cells, however, the mice still detected the stimulus and withdrew their paws, but they showed a reduced affective response to the painful stimulus. This suggests that the BLA cells encode the unpleasantness associated with pain.
BLA Cells Also Encode Aversive Effects of Chronic Pain
To determine whether the BLA cells also affected the experience of chronic pain, the investigators conducted additional experiments using mice with chronic pain caused by nerve damage (i.e., neuropathic pain). These animals typically become overly sensitive to stimuli such as light touch and cool temperatures that a normal mouse does not experience as painful, and therefore avoid them. However, when the cells in the BLA network were deactivated in mice with neuropathic pain, the animals no longer showed aversion to cool temperatures, indicating that they no longer experienced the unpleasant effects of excessive cold sensitivity (see Figure 2). Further experiments confirmed that the manipulations did not simply impair the animals’ ability to experience any aspect of pain as they still showed, for example, normal withdrawal reflexes.
Dr. Scherrer hopes that this research will ultimately help improve pain management in humans. He explains, “What we now want to do is look at these cells in greater detail and ask if we can find some targets to develop new pain medicines that would turn off the cells to reduce the unpleasantness of pain.” He adds, “We’re interested in finding targets that are present in these cells but are absent in regions that are, for example, causing addiction, so all the places that are associated with opioids’ actions. We are hoping to mimic the analgesia of opioids but without the side effects.”
Dr. Mark J. Schnitzer from Stanford University and Investigator of the Howard Hughes Medical Institute, who was also a Senior Investigator on the project, adds, “This biological finding was greatly facilitated by NIDA’s initial funding, provided almost two decades ago, of new technology that allows one to see activity in individual cells in the brain of a freely moving mouse.”
This study was supported by NIDA grants DA031777, DA043609, DA041029, and DA35165.
- Text Description of Figure 1
-
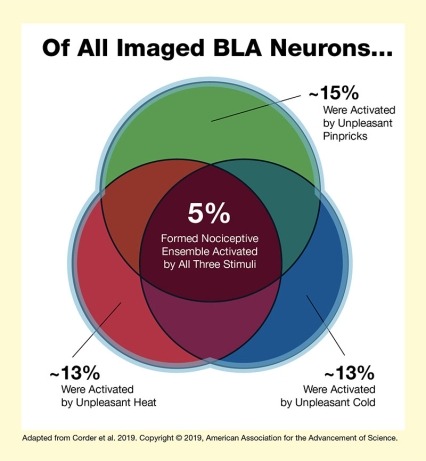
The figure graphically illustrates the concept that a small group of neurons in the BLA form a nociceptive ensemble that is activated by several unpleasant stimuli. The white background square represents all neurons in the BLA. A green circle at the top represents the 15 percent of BLA cells that were activated by unpleasant pinpricks. A red circle at the bottom left represents the 13 percent of BLA cells that were activated by unpleasant heat, and a blue circle at the bottom right represents the 13 percent of BLA cells that were activated by unpleasant cold. The three circles overlap, and the dark red shape in the center that is part of all three circles represents the 5 percent of BLA cells that form the nociceptive ensemble activated by all three unpleasant stimuli.
- Text Description of Figure 2
-
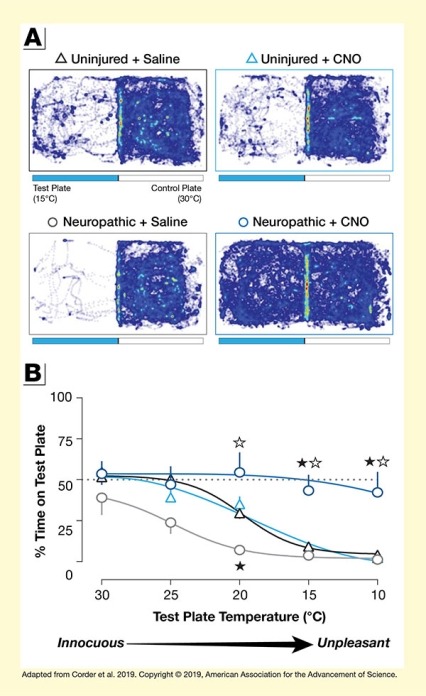
This figure illustrates findings that cells in the BLA mediate the aversive effects of chronic pain. Panel A at the top shows to what extend different groups of mice run freely across cool test plates and warm control plates. The figure has four panels for four different groups of mice. In each panel, the left half represents the cool test plate with a temperature of 15oC and the right half represents the warm control plate with a temperature of 30oC. Blue lines indicate the running tracks of the mice. The top left panel shows the running tracks of uninjured mice treated with saline. For these animals, there are few tracks on the test plate and very dense tracks on the control plate, indicating a preference for the warm plate.
The top right panel represents uninjured mice treated with clozapine-N-oxide (CNO) to inactivate the BLA network. Again, there are few tracks on the test plate and very dense tracks on the control plate. The bottom left panel represents mice with chronic neuropathic pain and treated with saline. There are hardly any tracks on the test plate and very dense tracks on the control plate, indicating that the mice avoided the test plate. The bottom right panel represents mice with neuropathic pain and treated with CNO. For these mice, there were dense tracks on both the cool test plate and the warm control plate, indicating that the animals did not find the cool plate unpleasant.
Panel B at the bottom shows line graphs illustrating how much of their time the four groups of animals spent on the test plate as its temperature was lowered from 30oC to 10oC. The horizontal x-axis shows the temperature of the test plate on a scale from 30oC (innocuous) to 10oC (unpleasant). The vertical y-axis shows the percent time spent on the test plate on a scale from 0 percent to 100 percent. A dotted horizontal line indicates the 50 percent mark. Uninjured mice treated with saline are represented by black triangles. They spent about 50 percent of their time on the test plate at 30oC and 25oC, about 30 percent of their time at 20oC, about 10 percent of their time at 15oC, and about 5 percent of their time at 10oC. Uninjured mice treated with CNO are indicated by blue triangles; their results are similar to those of uninjured mice treated with CNO. They spent about 50 percent of their time on the test plate at 30oC, about 40 percent of their time at 25oC, about 35 percent of their time at 20oC, about 10 percent of their time at 15oC, and almost 0 percent of their time at 10oC. Gray circles represent animals with chronic neuropathic pain treated with saline. They showed greater temperature sensitivity, spending about 40 percent of their time on the test plate at 30oC, about 25 percent of their time at 25oC, about 10 percent of their time at 20oC, about 5 percent of their time at 15oC, and almost 0 percent of their time at 10oC. Dark blue circles represent mice with chronic neuropathic pain and treated with CNO. They were barely affected by reduced temperatures, spending about 55 percent of their time on the test plate at 30oC, about 45 percent of their time at 25oC, about 55 percent of their time at 20oC, and about 40 percent of their time both at 15oC and at 10oC.
Source:
- Corder, G., Ahanonu, B., Grewe, B.F., et al. An amygdalar neural ensemble that encodes the unpleasantness of pain. Science 363(6424):276-281, 2019.